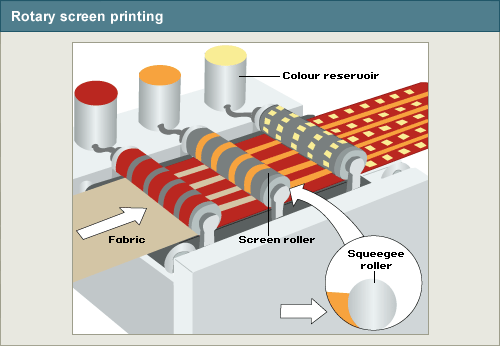
Given the large size of the printing industry, and the extraordinary volume of chemicals it consumes, it is not surprising that it also generates a significant amount of pollution. Gaseous emissions have been identified as the second greatest pollution problem (after effluent quality) for the textile industry – and these are largely generated in printing. Speculation concerning the amounts and types of air pollutants emitted from textile operations has been widespread but, generally, air emission data for textile manufacturing operations are not readily available. Air pollution is the most difficult type of pollution to sample, test, and quantify in an audit.[1] According to the U.S. EPA, the printing industry releases 99% of its total Toxic Release Inventory (TRI) poundage to the air, while the remaining one percent of releases are split between water and land disposal. This release profile differs significantly from other TRI industries which average approximately 60% to air, 30% to land, and 10% to water release respectively. Average VOC emissions per textile print line are 130 Mg (tons)/year for roller and 29 Mg/year for flat and rotary screen.[2]
In 1995, more than 41 million pounds of toxic compounds were transferred or released into the environment by the printing industry in the United States alone. The table below shows some of the polluting chemicals used by the textile printing industry. All ten are petroleum-derived.
| Chemical | Releases and transfers in millions of pounds |
| Toluene | 4.2 |
| Methyl Ethyl Ketone | 6.3 |
| Glycol Ethers | 0.4 |
| Xylene | 0.2 |
| Methyl Isobutyl Ketone | 0.6 |
| Methanol | 0.3 |
| 1,1,1-Trichloroethane | 0.3 |
| Ethylene Glycol | 0.5 |
| Dichloromethane | 0.1 |
Source: EPS: Profile of the Textile Industry, EPA/310-R-97-009, September 1997
These VOC emissions are high because of the great quantity of solvents used in the industry. The volatility that helps minimize ink drying times also presents a health and safety risk. The solvents used in the printing pastes are typically respiratory, skin and eye irritants. But there are also more dire consequences – for example, a study done on Indian printing working has found abnormal changes in their chromosomes.(3) With such a high percentage of the paste being volatile, solvent vapors will be released during printing and will be present throughout the printing production area. Also, the fabric will continue to off-gas solvents after the material has been printed, especially if it has been rolled up. The Sector Notebook gives a short synopsis of these chemicals, and I’ve excerpted a few here:
- Toluene, although used primarily as a solvent, is also used throughout printing for cleanup purposes. Toluene contributes to the formation of ozone in the atmosphere; studies have shown that unborn animals were harmed when high levels of toluene were inhaled by their mothers, although the same effects were not seen when the mothers were fed large quantities of toluene. Note that these results may reflect similar difficulties in humans.
- Data on ethylene glycol mono-n-butyl ether is used to represent all glycol ethers because it is the most commonly used glycol ether in printing. It can leach into ground water, and reacts with photochemically produced hydroxyl radicals. For humans, moderate exposure may cause central nervous system depression, including headaches, drowsiness, weakness, slurred speech, stuttering, staggering, tremors, blurred vision, and personality changes. These symptoms are such that a patient, in the absence of an accurate occupational history, may be treated for schizophrenia or narcolepsy.
- Methyl ethyl ketone contributes to the formation of air pollutants in the lower atmosphere; breathing “moderate amounts” for short periods of time can cause adverse effects on the nervous system ranging from headaches, dizziness, nausea, and numbness in the fingers and toes to unconsciousness; repeated exposure to moderate to high amounts may cause liver and kidney effects.
Everybody is now talking about “water based” inks, as if that’s the answer to help reduce these emissions. So, let’s investigate these inks and see what “water based” means, and what the concerns may be.
There are three general types of texile inks (or pastes, as we referred to them in Printing – Part 2):
- traditional solvent-based inks
- water-based inks
- plastisol inks
The two inks used most often in textile printing are water-based (used mostly for yardgoods) and plastisol inks (used for printing finished goods, such as T shirts, sweatshirts, tote bags).
SOLVENT-BASED INKS: The solvent has two primary functions: 1) to carry the ink to the substrate, and 2) to evaporate quickly, leaving only the ink film on the substrate. While water is a solvent, the name solvent-based ink is used to describe a highly volatile solvent such as 2-butoxyethyl acetate, cyclohexanone and n-butyl acetate.
Solvent based inks are considered the least environmentally friendly due to the highly volatile solvents given off during printing and drying. The petroleum-based binder used in many solvent-based inks could be replaced with renewable resources such as vegetable oil or soy. The downsides are that the inks dry very slowly are less durable, and still contain solvents emitting VOCs during printing.
Therre are now inks on the market called Eco Solvent inks. To most people, “eco” means ecological, and to be fair these inks are not as nasty as full solvent inks. But these inks generally contain glycol esters or glycol ether esters – both derived from mineral oil – hardly a renewable resource or an ecologically sound process. Tony Martin, president of Lyson Inc. suggested we call these inks “mild” vs. the “aggressive” traditional solvent inks. Also since these inks are generally used to print onto PVC, the green claim sorta gets overlooked by the elephant in the substrate.
WATER-BASED INKS: These use water as the main solvent. But that does not mean that water is the ONLY solvent used. It is significant to note that many water base inks contain “co-solvents” which may even be petroleum based solvents.[4] ( See Printing – Part 2 for components of typical water and solvent based inks.) The reason these co-solvents are used varies, but a main reason is to decrease the time and heat necessary to cure the ink on the fabric.
There are two types of water-based inks: Traditional (air dry) ink and Discharge ink.
- Traditional air dry ink soaks into the cloth and binds with the fibers providing good colorfastness and wash ability.
- Discharge ink removes the original dye/color from the garment and replaces it with a color/pigment. Discharge inks are now available in formaldehyde free formulations, such as the Oasis Series by Wilflex, making them safer for the user and the environment.
Water based inks are usually less expensive than solvent-based inks and are similar in quality, gloss, and adhesion.
Many printers observe that water-based inks have more vibrant colors and print more crisply than their solvent-based counterparts. The sharper definition possible with water-based inks allows printers to use finer dot patterns in screened process printing. Water-based inks are a good choice when a “soft hand” is desirable. (A soft hand is the condition where the ink film cannot easily be felt with the hand when passed across the surface of the fabric. This affect is often used as an argument for why water-based is preferable to plastisol because plastisol has more of a hand than water-based, and this is considered a consumer turn off.)
These inks are inexpensive and easy to manufacture. In fact, with some experience and the proper equipment, printers can even make them in small batches from basic natural components. They have a very limited shelf life and are difficult to re-use, so they generate more wasted ink than regular plastisols or more complex, manufactured water-based inks. While this type of water-based ink is considered a very green alternative, this extra waste is something to consider.
An advantage often cited for water-based inks is that they do not require organic solvents when cleaning the presses. But there is a common misconception that because water can be used for cleaning screens, squeegees and tools, that the waste water can just be discharged into the sewer. However, the water-based ink is not just water. There are pigments, binders, thickeners, and sometimes, even co-solvents in the ink residue.
Many printers believe that screen printing using water based inks is the cutting edge of textile printing. So why isn’t everybody using them?
Water-based inks cure as water evaporates out of the ink so they have a longer – and more difficult – drying time than plastisol inks. This means that the water — along with whatever in the ink evaporates with the water — enters the environment.
If using water-based ink, the facility must have the drying capacity to remove the water. The dryers used for water-based printing tend to be larger than those needed for plastisol. In plastisol printing, the ink film must only reach the cure temperature for a brief moment. With water-based ink, the temperature must be reached and then held until all of the solvent (water) is removed. There are water-based inks that will air dry but they are usually only acceptable for craft level printing as the room required for curing greatly reduces productivity.
Finally, all water-based inks can start to dry out during use, so care must be taken to prevent the ink from drying on the screen. If water based ink is left in open mesh for even a short period of time, it can clog the mesh and ruin the screen. Practiced waterbased ink printers must always be conscious of how long a screen sits between prints to prevent the ink from “drying in”. While modern water-based inks are less prone to this phenomenon, it is still a concern. In addition, overall shelf life is limited.
There have been major improvements in manufactured water-based inks in recent years. These newer inks have a number of performance advantages over the basic water-based inks discussed above and are as potentially eco-friendly and sustainable as any alternative. For example, they resist drying, and remain useable far longer than traditional water-based and discharge inks. They can be re-constituted with water — and additional binder, if needed — which can cut back on waste. Shelf life of these newer water-based inks is substantially longer as well because the manufacturers have developed technology to encapsulate the water in the ink in such a way that it does not readily evaporate until printed.
Much like traditional plastisol, these water-based inks are sold ready to use as colors or underbases and have a thicker viscosity that yields greater opacity on finished prints. They can be reduced with water and other modifiers for a softer hand.
PLASTISOL INKS: Plastisol inks, commonly used for textile printing and especially for t-shirts, are a PVC-based ink composed of a clear, thick plasticizer fluid and PVC resin. The full name for PVC is polyvinyl chloride. The PVC life cycle results in the release of toxic, chlorine-based chemicals which end up as by-products such as carcinogenic and highly toxic dioxin and PCB. The major health concern about plastisol inks is not that they are PVC-based but that they contain phthalates. Phthalates are added to PVC plastics to transform a hard plastic into a soft, rubbery plastic by allowing the long polyvinyl molecules to slide against each other instead of rigidly binding together. These phthalates used in plastisol ink to make the PVC flexible are also carcinogenic and much research has been done which substantiates the damage phthalates do to us, especially to fetuses and newborns.[5] They are released into the environment during the printing and curing of the ink and they will continue to exhaust toxins when exposed to a radiant heat source, such as a dryer or even sunlight. Plastisol inks contains virually no solvents at all.
Plastisol does not “dry”. In order for a compound to dry, there must be evaporation of some kind of solvent. These inks typically contain less than 1% VOC. Some water based plastisol inks can contain about 30% VOCs.[6] Since plastisol has little or no solvent, it cannot dry. Plastisol is a thermoplastic ink – meaning it is necessary to heat the printed ink film to a temperature high enough to cause the molecules of PVC resin and plasticizer to cross-link (i.e., bond to the fabric) and solidify, or cure. Cross-linking agents must be used to effect the bonding, and formaldehyde is often a necessary component of these cross linkers. The temperature at which most plastisol for textile printing cures at is in the range of 300 °F to 330 °F. Because of this characteristic, plastisol can be left in screens for long periods of time without clogging the mesh, the lids can be left off of the ink containers (although keeping them covered is a good practice to keep lint and dirt out of the ink). And ink left at the end of the job can be returned to the container for reuse without any adverse affects. This last practice is a great benefit in reducing waste product. It is ready to use right out of the container more than 90% of the time. In most applications, it can be printed wet-on-wet, which allows for increased production speeds. It comes in formulations that can be printed on light and dark fabrics.
Since Plastisol is a thermoplastic, it will remelt if it comes in contact with anything hot enough. For that reason, plastisol prints cannot be ironed. If an iron touches a print, it will smear the ink.
Plastisol ink also creates an ink film that can be felt with the hand. The higher the opacity of the ink, the greater the hand. This heavy hand is considered a disadvantage at the consumer level.
Because both PVC and phthalates are chemicals of concern, many companies are offering phthalate free plastisol inks. These non-phthalate inks are not as easy to work with as standard plastisols, but it is possible to use them to accomplish most of the common printing techniques. In addition to non-phthalate plastisols, there are some new acrylic-based screen printing inks that are sometimes referred to as non-PVC and non-phthalate plastisols. Why? Well, an acrylic-type resin replaces the PVC resins used in regular plastisol. Also, the plasticizer in acrylic inks is normally non–phthalate, making these inks an even more eco-friendly alternative.
With some experience, acrylic inks can be successfully made into high-density designs. The finished prints lack the soft finish of a standard high-density plastisol print, but this may be an acceptable compromise to some customers.
Acrylic inks are usually a little more costly than standard plastisols and are substantially more expensive than standard water-based inks.
The hazards of plastisol printing inks are not just to personal health but also to environmental health. Garments coated with plastisol inks do not decompose and they are difficult to recycle. The result is that you may soon grow tired of your Rolling Stones concert tee shirt and trash it, but it will live on in immortality in the local landfill. If clothing designed with PVC plastisol ink is incinerated, the trapped dioxins plus hydrochloric acid (a primary component of acid rain) are released into the atmosphere.
New inks have also been developed for digital printing, such as latex, resin and UV curable inks. We’ll discuss them next week with digital printing.
Dr. Nicholas Hellmuth, of FLAAR (http://www.wide-format-printers.org/), writing in his January, 2011 blog, said of the proliferation of green claims by ink manufacturers: ” I would bet that 90% of these claims were misleading at best. I would bet that more than 50% of these claims are fraudulent and inaccurate… I looked at the MSDS of inks called water-based and almost gagged when I saw the chemical recipe, with the hazardous warnings. If you make a list of the nasty chemicals that are really in the ink, depending on what chemicals you consider unhealthy, resin ink could potentially be considered less unhealthy than even traditional water-based ink. In other words, there is a potential that resin inks could be considered better than water-based inks. But there are so many diverging opinions that I will be discussing this with other ink chemists as I meet them during the expos early in this year (2011). ”
So you’d think that the major source of the emissions comes from using these inks – the printing process itself. You’d be wrong: the majority of emissions to the atmosphere from textile printing is from the drying process, which drives off volatile compounds. The largest VOC emission source is the drying and curing oven stack, which vents evaporated solvents to the atmosphere. Another source of fugitive VOC emissions comes from the “back grey” (fabric backing material that absorbs excess print paste), which is dried before being washed. In processes where the back grey is washed before drying, most of the fugitive VOC emissions from the back grey will be discharged into the waste water. In some roller printing processes, steam cans for drying printed fabric are enclosed, and drying process emissions are vented directly to the atmosphere.
As of the publication date of the EPA Sector notebook on the Printing and Publishing Industry (1995), there was no add-on emission control technology for organic solvents used in the textile printing.
Another environmental hazards in printing textiles comes in the screen and equipment cleaning steps – which use lots of water. When you finish a printing run, for example, there are still approximately 1.5 gallons of printing paste in the system, predominantly in the tubes that run between the paste reservoirs and the screens. This is simply rinsed out and flushed down the drain. If using plastisol inks, in order to emulsify the ink for easy removal from screens, squeegees, flood bars, spatulas, and work surfaces, it is necessary to use some type of solvent. Solvents used to clean printing equipment include toluene, xylene, methanol, and methyl ethyl ketone (MEK). In addition, blankets used to transfer the ink-filled image to sheets of paper are cleaned with washes that contain glycol ethers and 1,1,1-trichloroethane (TCA). The type of solvent used depends largely on the equipment to be cleaned. For example, a blanket wash must dissolve ink quickly and dry rapidly with minimal wiping. Conversely, a solvent that is intended to clean a chain of ink rollers must evaporate slowly, to insure that it does not flash off before it has worked its way through all the rollers. Water based inks contain co-solvents, additives, dyes and/or pigments, which make the water clean up full of possibly hazardous materials. All of these components must be washed thoroughly.
Irrespective of the type of inks used, all printers attempt to reclaim screens, which are a major cost item. Failure to reclaim screens and ruined screens cost on average $5,000-$10,000 per year. One study showed chemical reclamation cost between $2 and $10 per average screen, while screen disposal cost just shy of $50. Screen reclamation is a particular challenge to screen printers, because inks and solvents cannot go down the drain and some of the chemicals used to reclaim mesh are restricted. The waste water will contain particulates comprised of ink pigment, emulsion and emulsion remover. Reclaiming screens involves these steps:
- Remove the paste: Any and all excess paste in the screen should be “carded off” for reused on another job. The screen must then be washed to remove any remaining paste because the paste will interfere with the process of removing the stencil. Screen cleaning solvents are a source of VOC emissions.
- Emulsion removal: The stencil or emulsion is removed by spraying the screen with a solution of water and emulsion remover chemicals which is comprised mainly of sodium metaperiodate, then rinsing the solution away with fresh water.
- Haze or ghost image removal: Finally, if any haze or “ghost image” remains, a haze remover must be applied. Some haze remover products are caustic and can damage or weaken the screen. Haze removers make screens brittle and tear easily, therefore only small amounts should be used. Ghost image is a shadow of the original image that remains on the screen caused by paste or stencil caught in the threads of the screen.
The best way to reduce VOCs during screen reclamaition are related to technology and best practices, such as using high pressure wash systems and modifying how chemicals are applied to the screens.
The waste ink and the solvent must be disposed of properly in order to minimize environmental impact. There are three major areas of concern for this wastewater:
- Heavy metals, which can be found in the residue of ink, can enter the sewer system and contaminate sewage sludge
- Heavy concentrations of certain chemicals can disrupt the pH balance at the treatment plant and disrupt the bacterial systems essential to the sewage treatment process
- Combinations of mixtures with low flash points can cause flammability concerns in the sewage system
Leftover print pastes cannot be allowed to enter the wastewater treatment system. It must be disposed of as a solid waste. Sites where sludge piles are used can have environmental problems with ground and groundwater contamination. These sludge storage areas should be equipped with waterproof linings to prevent this from occurring.
In fact, textile printing is becoming an important wastewater source as the water-based materials replace the organic solvents. The wastewaters originating from this operation are often strong and may contain toxics, although their volume is still quite low.[7]
The screen printing industry has been very proactive in the creation of products that can minimize the impact of these cleaning processes. Solvents are available that are “more” environmentally sensitive than the traditional petroleum based solventsCompanies are beginning to market biochemical cleaning solutions, inks and additives to replace current solvents or toxic chemicals– examples include the use of terpene d-limonene (derived from citrus fruit), coconut oil , soybeans, seaweed and fatty amides. (8) In addition, there are many types of filtration and cleaning systems available to capture inks and solvent residues to minimize the solids that are discharged into the sewer system.
Aside from improvements to the building itself and efforts to minimize water use and to use inks and paste effectively, there are some things every printer can do to reduce their environmental impact:
- Minimize downtime on the press
- Make rejects history
- Maintain dryers – is it really worth saving money by buying that second hand dryer? A new one is 30% more efficient, twice the price but the energy savings will pay the difference in 9 months. An average printing line has a nominal power rating of 75 kW, most of which is required for the drying process.
[1] The Indian Textile Journal, http://www.indiantextilejournal.com/articles/FAdetails.asp?id=2420
[2] http://www.epa.gov/ttnchie1/ap42/ch04/final/c4s11.pdf
(3) Sellappa, Sudha, et al; Genotoxic Effects in Textile Printing Dye Exposed Workers by Micronucleus Assay, Asian Pacific Journal of Cancer Prevention, Vol 11, 2010; pgs. 919-922, http://www.apocp.org/cancer_download/Volume11_No4/c%20919-22%20Sellappa.pdf
[7] Kabdasli, M Gurel & Tunay, O., “Characterization and Treatment of Textile Printing Wastewaters”, Environmental Technology, Vol 21, Issue 10, 2000, pp. 114 – 1155
(8) http://www.pneac.org/sheets/all/biochemicals_for_the_printing_industry.pdf